
Function of the estrobolome
The gut microbiome encodes a vast number of enzymes that function in a variety of metabolic pathways, including the biosynthesis of essential nutrients, the breakdown of complex carbohydrates and the biotransformation of metabolic products such as conjugated estrogens. The estrobolome contributes to estrogen homeostasis where both elimination and recycling help to maintain a healthy balance of estrogens. Estrogens also regulate the gut microbiome in a positive manner by increasing the diversity of the gut microbiota and augmenting the enzymes that metabolize estrogens (3).
Interactions between the human host and microbes have the potential to influence carcinogenesis through mechanisms such as chronic inflammation, induction of genotoxic responses, and alteration of the microenvironment where this interface occurs (4). Estrogens can impact the gut microbiota to support immune function, regulate inflammation, and influence hormone-dependent cancers especially after menopause. While the reactivation of estrogen metabolites may serve specific functions, it has also been hypothesized that a woman’s estrobolome plays an influential role in the development of several hormonal disorders, including breast, endometrial, and ovarian cancers (2).
Liver metabolism and estrogens
Estrogens are primarily produced by the ovaries in premenopausal women and by the adrenal glands and adipose tissue in postmenopausal women. They circulate in the bloodstream in free or protein-bound forms and undergo metabolism primarily in the liver where they are converted to inactive metabolites through Phase I and Phase II liver detoxification pathways (4). Phase I liver detoxification involves cytochrome P450 enzyme pathways, converting estrogens into more water-soluble compounds.
Phase II liver detoxification conjugates (sulfation and glucuronidation), oxidizes, reduces, and/or methylates estrogen metabolites from Phase I liver detoxification. The conjugation of estrogens to glucuronic acid specifically marks the estrogen-glucuronide for elimination where it eventually passes through the kidneys for elimination through the urine or is moved out of the liver through the bile where it is ultimately released into the bowel for elimination through the stool. Conjugated estrogens excreted in the bile can be deconjugated by β-glucuronidase enzymes produced by resident bacteria in the intestines. This subsequently leads to estrogen reabsorption through enterohepatic circulation and ultimately enables estrogens to enter target tissues, where they bind to and activate estrogen receptors (4).
Gut enzymes and estrogen metabolism
The gut microbiome is a principal regulator of circulating estrogens (5). There are 279 β-glucuronidases that have been identified in the Human Microbiome Project and they are produced by a variety of normal gut microbial species and have varying degrees of activity. Additionally, sulfatase enzymes, though less characterized than β-glucuronidases, also play a role in estrogen metabolism. Gut microbial sulfatases process sulfated forms of estrogens and DHEA (6).
A bidirectional regulatory system between β-glucuronidases and estrogens exists to maintain estrogen homeostasis in the body (7). Beyond simple reactivation of estrogens, the estrobolome acts as an estrogen reservoir in the gut and is capable of creating estrogenic metabolites for local and nonlocal functions (2). Estrogens regulate the gut microbiome in a positive manner by increasing microbial diversity. Increased microbial diversity is associated with decreased production of β-glucuronidases, allowing for greater excretion of conjugated estrogens.
When systemic estrogens are low, as in perimenopause or menopause, microbial diversity decreases and production of β-glucuronidases increases, potentially allowing for enhanced estrogen recycling (3). Though β-glucuronidases aid in the regulation of local and systemic levels of estrogens, an overabundance of these enzymes in the gut and other tissues may contribute to a state of estrogen dominance, where estrogen levels are high relative to progesterone and cause excessive stimulation of growth of estrogen sensitive tissues such as the breasts and uterus.
Signs of estrogen dominance include:
- Fibrocystic breasts
- Uterine fibroids
- PMS/PMDD
- Mood swings/anxiety/depression
- Hormone-related headaches
- Heavy menses/irregular cycles
- PCOS
- Infertility
- Endometrial hyperplasia
- Breast cancer
- Weight gain and water retention
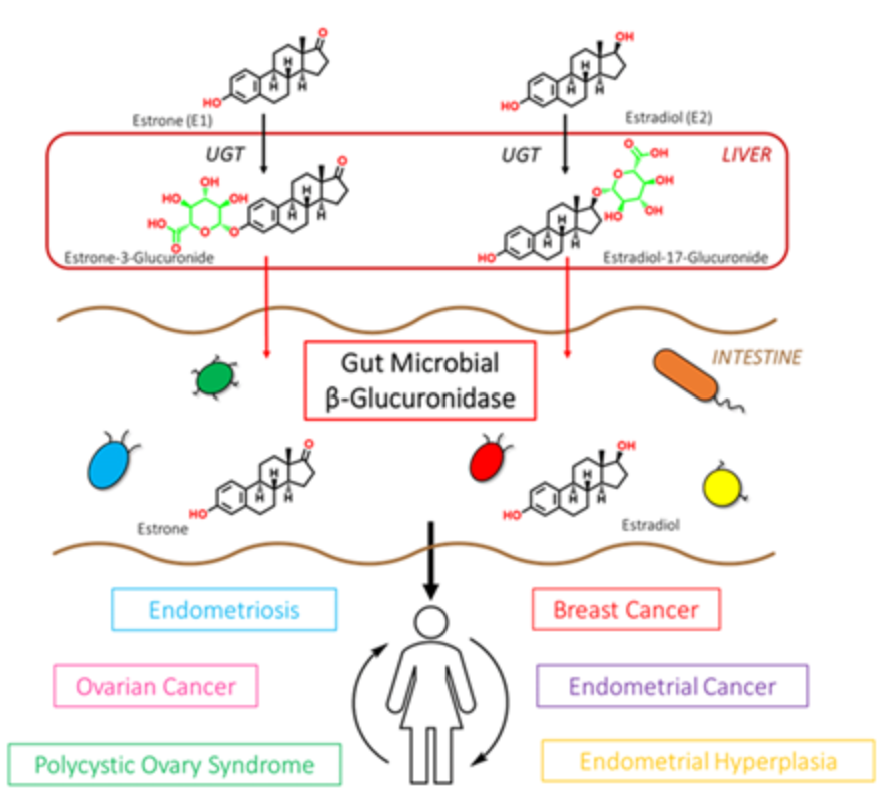
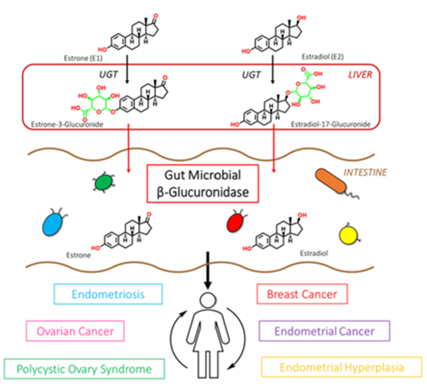
β-glucuronidase enzymes reactivate estrogens. Gut microbial β-glucuronidase enzymes within the GI deconjugate estrone-3-and estradiol-17-glucuronides to the aglycones estrone and estradiol, respectively. This reactivation allows unbound estrogens to be recirculated through the bloodstream, possibly contributing to a variety of hormonal disorders including breast cancer and endometriosis. Ervin SM, Li H, Lim L, et al. Gut microbiome–derived β-glucuronidases are components of the estrobolome that reactivate estrogens. J Biol Chem. 2019;294(49): jbc.RA119.010950. Creative Commons licensing.
Balanced gut bacteria and the estrobolome
Bacteroidetes and Firmicutes, the main phyla dominant within the GI tract, are the primary source of β-glucuronidases. A higher ratio of Firmicutes to Bacteroidetes may be indicative of a high-fat diet in which saturated fat from meats has the greatest effect on promoting bacteria that produce β-glucuronidases. As noted by Sui et al, a considerable number of studies have linked a high-fat diet with increased β-glucuronidase activity. Additionally, a higher ratio of Firmicutes to Bacteroidetes is linked to obesity, which predisposes one to several chronic diseases, including cancer. The β-glucuronidases produced from the Firmicutes phyla of bacteria have the highest level of estrogen reactivation as compared to those from the Bacteroidetes phyla (7).
What about androgens and progesterone?
You’re probably asking– what about an androbolome or a progestobolome? There are some studies showing that androgens and progesterone interact with the gut microbiome in a bidirectional manner, but it is less well characterized than the estrobolome. Changes in the gut microbiome when testosterone and progesterone are increased have been demonstrated, and we can see the systemic effects of that relationship (8, 9).
In the liver, testosterone is metabolized similarly to estrogens that are hydroxylated by Phase I enzymes in the liver and then glucuronidated or sulfated in Phase II. The testosterone conjugates are then absorbed and excreted through the urine or expelled through the bile into the intestines. The β-glucuronidase enzymes can act on testosterone-glucuronides just as these enzymes can act on estrogen-glucuronide, resulting in the freeing of testosterone to be reabsorbed systemically (10). This familiar mechanism can result in increased systemic testosterone with increased β-glucuronidase levels in the gut, and would potentially free testosterone for reabsorption.
Progesterone metabolism is more complex, resulting in multiple metabolites formed through sequential enzymatic reduction pathways that ultimately form pregnanediols. In Phase I reactions, progesterone is converted primarily to pregnanediol that is converted to pregnanediol-glucuronide. Progesterone is also metabolized to allo-pregnanolone, a neuroactive molecule that freely enters the brain and has a calming effect through its interaction with GABA-A receptors (11). Removal of the glucuronide in the gut transforms pregnanediol-glucuronide back to pregnanediol, which is inert and does not have the anti-estrogenic activity of progesterone. This would mean that gut metabolism of glucuronides of estradiol and pregnanediol would lead to an overabundance of estradiol without the protective actions of progesterone.
Qi et al have noted changes in the gut microbiota of pregnant women which were linked to differences in metabolic, immunological, and hormonal variations. High progesterone levels are essential for a healthy pregnancy and fetal development. In turn, dramatic shifts in hormone levels also impact gut function and bacterial composition, accompanied by unique inflammatory and immune changes that are supportive of pregnancy (11).
Seeing the unseen
Stool testing can help us to ‘see the unseen’ by examining the various species of bacteria in the gut. We can also review functional markers of digestion, inflammation, mucosal immunity, and deconjugating enzymes such as β-glucuronidase. Poor digestion of proteins, carbohydrates and fats can contribute to bacterial imbalances throughout the GI tract and may indicate the need for digestive support and functional evaluation of the liver, biliary tract, and pancreas.
Elevated inflammatory markers may be indicative of infections, food sensitivities and intolerances, inflammatory bowel disease, and cancer. Elevated markers of mucosal immunity may indicate infection and inflammatory processes that can eventually lead to gut permeability (leaky gut) and systemic disease and inflammation. Low levels of mucosal immune markers indicate poor resistance to infection, microbial imbalances, and chronic stress. A high level of β-glucuronidase enzymes on a stool test is associated with dysbiosis and may increase circulating levels of estrogens, mostly estrone and estradiol.
Testing of saliva and blood for estrogens, progestogens, and androgens and urine for steroid hormone metabolites should help determine the source of estrogen dominance that may be precipitated by gut dysbiosis.
What exactly is dysbiosis?
Dysbiosis is a broad term used to describe an imbalance in bacterial composition, changes in bacterial metabolic activities, or changes in bacterial distribution within the gut (12). Dysbiosis disrupts homeostasis by reducing microbial diversity and is associated with increased oxidative stress, inflammation, and damage to DNA repair mechanisms (3). Dysbiosis may also increase the Firmicutes to Bacteroidetes ratio which can lead to an inflammatory state that is detrimental to the health of gut epithelial cells and can compromise gut barrier integrity, leading to intestinal permeability and bacterial translocation (5). Lipopolysaccharides (LPS) are components of gram-negative bacteria that, when systemically absorbed through a permeable gut membrane, can trigger inflammation and induce systemic diseases such as insulin resistance, PCOS, and metabolic syndrome (11).
The three main types of dysbiosis and support options include:
- Loss of beneficial bacteria or deficiency dysbiosis as is often seen with antibiotic use and reduced consumption of a wide variety of plant-based foods. Support with prebiotics, probiotics, increased consumption of plant-based foods and sources of fiber.
- Overgrowth of potentially pathogenic bacteria as seen with co-infections (microbial, parasitic, fungal) and high-carb, high-fat diets. Support with botanical antimicrobials, probiotics, binders, digestive enzymes, fiber and treatment of underlying infections.
- Loss of overall bacterial diversity as seen with poor diet, disease, antibiotics, and chronic gut inflammation. Support with prebiotics, probiotics, increased consumption of plant-based foods and sources of fiber along with regular exercise.
Supporting a healthy estrobolome
Diet, lifestyle, and robust elimination support a healthy estrobolome and keep β-glucuronidase and sulfatase enzymes within a healthy range to maintain a homeostatic state between estrogen elimination and estrogen reactivation. Excessive alcohol, sugar, processed foods, antibiotics, a lack of physical activity, and exposure to chemical toxins are contributors to microbial imbalance. A diet rich in fiber and resistant starches that feed the good bacteria in the GI tract are key to supporting a healthy balance of good bacteria and short-chain fatty acids, as well as keeping the bowels regular.
Daily elimination of estrogens through the gut and urine is necessary for the clearance of end-products of detoxification. The longer the estrogen conjugates from Phase II liver detoxification reside in the bowel, the greater the opportunity for deconjugating enzymes to act on these products and release excessive estrogens back into the systemic circulation. In addition to supporting the clearance of hormones, glucuronidation also participates in the elimination of neurotransmitters, thyroid hormones, bilirubin, chemical toxins including Bisphenol-A, drugs, mycotoxins, and other carcinogens (13).
Addressing underlying infections and dysbiosis by employing the use of botanicals, prebiotics, probiotics, and a diet weighted towards a variety of plant-based foods is supportive of gut health and a balanced estrobolome. Additionally, spending time in nature, with pets, and gardening are activities that are supportive of developing a healthy and diverse microbiome that gives back to us on multiple levels. As we learn more about the bacterial composition and function of our various microbiomes, we can understand the importance of supporting and optimizing their various roles in keeping us healthy.
Monitoring hormones
Correlating symptoms of estrogen excess with measured hormone levels provides objective data that can be monitored over time as efforts are made to improve estrogen metabolism through liver and microbiome support. Symptoms of estrogen excess or deficiency may be related to how efficiently hormones are cleared from the body. Estrogen clearance and recycling that occurs via the enzymes produced by the estrobolome can impact the systemic effects of estrogen. ZRT Laboratory offers a variety of tests to evaluate hormone levels either through dried urine, salivary, or dried blood spot sampling and can also be utilized to monitor efforts to create balance between estrogen elimination and recycling.
Related Tests
Female Hormone Test Profile II
References
- Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10(4):324-335.
- Ervin SM, Li H, Lim L, et al. Gut microbial β-Glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J Biol Chem. 294(49):18586-18599.
- Lephart ED, Naftolin F. Estrogen action and gut microbiome metabolism in dermal health. Dermatol Ther. 12(7):1535-1550.
- Kwa M, Ruggles KV, Novik Y, et al. Evaluation of the gut microbiome and sex hormones in postmenopausal women with newly diagnosed hormone receptor-positive breast cancer versus healthy women: a prospective case-control study. March 2022;1-25. DOI: 10.21203/rs.3.rs-144733/v1.
- Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas. 2017;103:45-53.
- Ervin SM, Simpson JB, Gibbs ME, et al. Structural insights into endobiotic reactivation by human gut microbiome-encoded sulfatases. Biochemistry. 2020;59(40):3939-3950.
- Sui Y, Wu J, Chen J. The role of gut microbial β-Glucuronidase in estrogen reactivation and breast cancer. Front Cell Dev Biol. 2021;9:631552.
- Ohayon MN, Belovgoski A, Komissarov S, et al. Progesterone supplementation in mice leads to microbiome alterations and weight gain in a sex-specific manner. bioRxiv. 2021. DOI: 10.1101/2021.10.06.463337.
- Hussain T, Murtaza G, Kalhoro D, et al. Relationship between gut microbiota and host-metabolism: emphasis on hormones related to reproductive function. Anim Nutri.2021;7(1):1-10.
- Li CY, Basit A, Gupta A, et al. Major glucuronide metabolites of testosterone are primarily transported by MRP2 and MRP3 in human liver, intestine and kidney. J Steroid Biochem Mol Biol. 2019;191:105350.
- Qi X, Yun C, Pang Y, et al. The Impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes. 2021;13(1):1-21.
- Clutter C. Disappearance of the human microbiota: how we may be losing our oldest allies. American Society for Microbiology. November 17, 2019.
- Watson B. What is glucuronidation? Xcode Life. August 31, 2021.

